Law of Triads
The development of the periodic table begins with German chemist Johann Dobereiner (1780-1849) who grouped elements based on similarities. Calcium (atomic weight 40), strontium (atomic weight 88), and barium (atomic weight 137) possess similar chemical prepares. Dobereiner noticed the atomic weight of strontium fell midway between the weights of calcium and barium:
Ca Sr Ba (40 + 137) ÷ 2 = 88
40 88 137
Was this merely a coincidence or did some pattern to the arrangement of the elements exist? Dobereiner noticed the same pattern for the alkali metal triad (Li/Na/K) and the halogen triad (Cl/Br/I).
Li Na K Cl Br I
7 23 39 35 80 127
In 1829 Dobereiner proposed the Law of Triads: Middle element in the triad had atomic weight that was the average of the other two members. Soon other scientists found chemical relationships extended beyond triads. Fluorine was added to Cl/Br/I group; sulfur, oxygen, selenium and tellurium were grouped into a family; nitrogen, phosphorus, arsenic, antimony, and bismuth were classified as another group.
Döbereiner’s classification scheme was improved and perfected by Mendeléev.
Law of Octaves
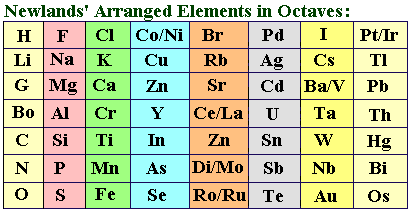
English chemist John Newlands (1837-1898), having arranged the 62 known elements in order of increasing atomic weights, noted that after interval of eight elements similar physical/chemical properties reappeared. Newlands was the first to formulate the concept of periodicity in the properties of the chemical elements. In 1863 he wrote a paper proposing the Law of Octaves: Elements exhibit similar behavior to the eighth element following it in the table.
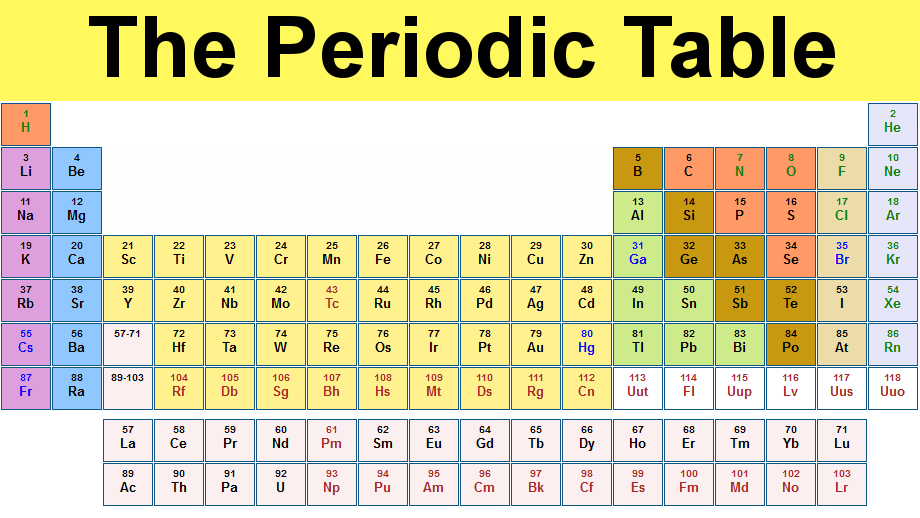
Mendeleev’s Periodic Table
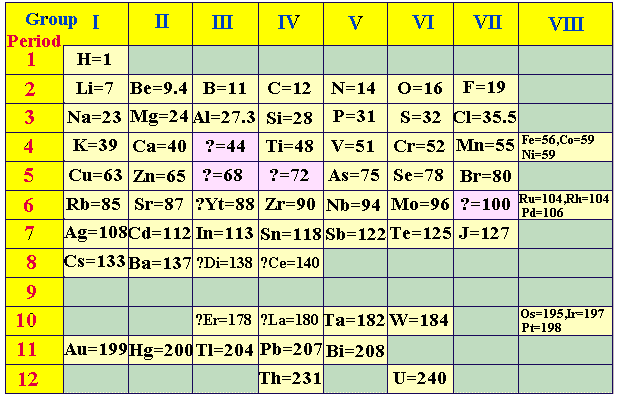
Then in 1869, Russian chemist Dimitri Mendeleev (1834-1907) proposed arranging elements by atomic weights and properties (Lothar Meyer independently reached similar conclusion but published results after Mendeleev). Mendeleev’s periodic table of 1869 contained 17 columns with two partial periods of seven elements each (Li-F & Na-Cl) followed by two nearly complete periods (K-Br & Rb-I).
In 1871 Mendeleev revised the 17-group table with eight columns (the eighth group consisted of transition elements). This table exhibited similarities not only in small units such as the triads, but showed similarities in an entire network of vertical, horizontal, and diagonal relationships. The table contained gaps but Mendeleev predicted the discovery of new elements. In 1906, Mendeleev came within one vote of receiving the Nobel Prize in chemistry.
Mendeléev, Dmitri (1834-1907)
Russian chemist who arranged the 63 known elements into a periodic table based on atomic mass, which he published in Principles of Chemistry in 1869. This organization surpassed attempts at classification by Beguyer de Chancourtois and Newlands and was published a year before the work of Lothar Meyer. Mendeléev left space for new elements, and predicted three yet-to-be-discovered elements including eka-silicon and eka-boron. His table did not include any of the noble gases , however, which had not yet been discovered. His table placed elements in their correct position by atomic number, thus showing variance from atomic weight in a number of places.
Moseley’s Periodic Law
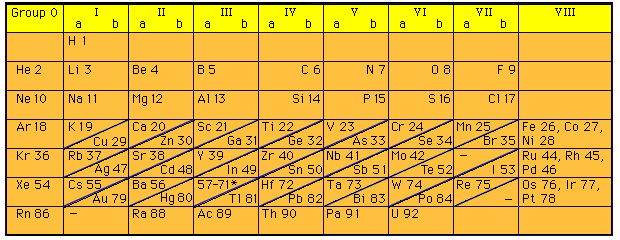
Soon after Rutherford’s landmark experiment of discovering the proton in 1911, Henry Moseley (1887-1915) subjected known elements to x-rays. He was able to derive the relationship between x-ray frequency and number of protons. When Moseley arranged the elements according to increasing atomic numbers and not atomic masses, some of the inconsistencies associated with Mendeleev’s table were eliminated. The modern periodic table is based on Moseley’s Periodic Law (atomic numbers). At age 28, Moseley was killed in action during World War I and as a direct result Britain adopted the policy of exempting scientists from fighting in wars. Shown above is a periodic table from 1930:
The Beacon website:
At The Beacon, we have created a Periodic Table of Technology to teach about science and technology by showing how elements are used in everyday tech-use. The interactive guide is a supplement to the other learning resources.
See The Periodic Table of Technology website here.

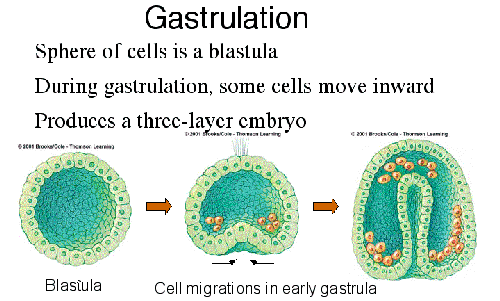
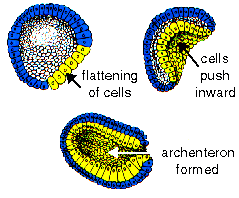 During gastrulation, cell movements result in a massive reorganization of the embryo from a simple spherical ball of cells, the blastula, into a multi-layered organism. During gastrulation, many of the cells at or near the surface of the embryo move to a new, more interior location.
During gastrulation, cell movements result in a massive reorganization of the embryo from a simple spherical ball of cells, the blastula, into a multi-layered organism. During gastrulation, many of the cells at or near the surface of the embryo move to a new, more interior location. The photosphere is the visible surface of the Sun that we are most familiar with. Since the Sun is a ball of gas, this is not a solid surface but is actually a layer about 100 km thick (very, very, thin compared to the 700,000 km radius of the Sun). When we look at the center of the disk of the Sun we look straight in and see somewhat hotter and brighter regions. When we look at the limb, or edge, of the solar disk we see light that has taken a slanting path through this layer and we only see through the upper, cooler and dimmer regions. This explains the “limb darkening” that appears as a darkening of the solar disk near the limb.
The photosphere is the visible surface of the Sun that we are most familiar with. Since the Sun is a ball of gas, this is not a solid surface but is actually a layer about 100 km thick (very, very, thin compared to the 700,000 km radius of the Sun). When we look at the center of the disk of the Sun we look straight in and see somewhat hotter and brighter regions. When we look at the limb, or edge, of the solar disk we see light that has taken a slanting path through this layer and we only see through the upper, cooler and dimmer regions. This explains the “limb darkening” that appears as a darkening of the solar disk near the limb.