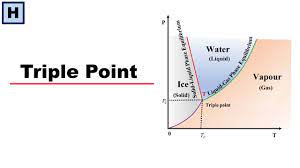
The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. As a result, at the triple point of water, the entropy is constant. Therefore, the entropy is constant at the triple point of water. The triple point of water is used to define the Kelvin(K), the base unit of thermodynamic temperature in the International System of Units (SI).
The third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches absolute zero. The entropy of a system at absolute zero is typically zero, and in all cases is determined only by the number of different ground states it has.
The single combination of pressure and temperature at which liquid water, solid ice, and water vapor can coexist in a stable equilibrium occurs at exactly 273.1600 K (0.0100 °C; 32.0180 °F) and a partial vapor pressure of 611.657 pascals (6.11657 mbar; 0.00603659 atm).